Your treatment journey
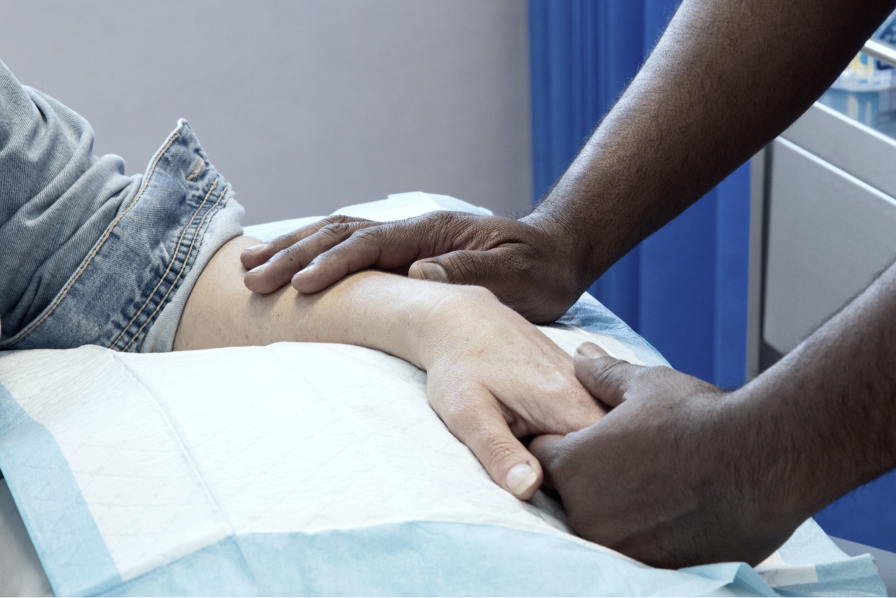
Your care at CRSA will be holistic and patient centred.
CRSA is located in a purpose-built facility overlooking the beautiful South Parklands, right alongside St Andrew’s Hospital in the CBD, with free street parking available close to CRSA.
Rights
CRSA respects the rights of people undergoing treatment, a full list of rights is available below.
Responsibility
CRSA believes that mutual trust, respect, and cooperation are basic to the delivery of quality health care. See full statement of Patients’ responsibilities below.
Safety
Quality in healthcare means the best possible health outcomes given the available circumstances and resources, consistent with patient centred care. See full statement of safety and quality below.
Our location
Cancer Research SA
L3, 337 South Terrace,
Adelaide SA 5000
Our facilities
CRSA is located in a purpose-built facility overlooking the South Parklands, right alongside St Andrew’s Hospital in Adelaide.
This location allows for convenient access to other specialists and healthcare options.
Parking
Free street parking is available close to CRSA.
Public transport
Please visit: www.adelaidemetro.com.au or Google Maps to plan your public transport route to CRSA.
Regional and remote treatment options
CRSA is working closely with Australian Teletrial Program, established by the South Australian Department for Health and Wellbeing to improve access for rural and remote patients to state-of-the-art trials.
Ask your doctor for more information or visit www.saclinicaltrials.sa.gov.au and www.cancersa.org.au/research
Wheelchair access
CRSA is fully accessible from South Terrace via the lifts in the main lobby.
Please call us on (08) 8359 2565 if assistance is required.
Dining nearby
Hutt St, a few minutes walk from CRSA, has multiple dining options from cafes and restaurants to IGA supermarkets selling ready meals and ingredients – www.cityofadelaide.com.au/blog/fringe-feasting-on-hutt-street and huttst.com.au
Accommodation
There are many options for overnight or longer stays close to CRSA in the Adelaide CBD, from major hotels to smaller or apartment-style accommodation.
Cancer Council SA also provide accommodation services for visiting patients. Find out more at www.cancersa.org.au/support/cancer-council-greenhill-lodge-accommodation/on-site-support-and-services
FAQS
Clinical Trials
What are clinical trials?
Clinical trials are research studies in which people help doctors and researchers find ways to improve health and cancer care. Each study tries to answer scientific questions and to find better ways to prevent, diagnose, or treat cancer. Clinical trials try to find causes of cancer, prevention of cancer, cancer screening and diagnosis, treating cancer as well as reducing side effects.
Why are there clinical trials?
A clinical trial is one of the final stages of a long and careful cancer research process. Studies are done with cancer patients to find out whether promising approaches to cancer prevention, diagnosis, and treatment are safe and effective.
What are the different types of clinical trials?
Treatment trials test new treatments (like a new cancer drug, new approaches to surgery or radiation therapy, new combinations of treatments, or new methods such as gene therapy).
Prevention trials test new approaches, such as medicines, vitamins, minerals, or other supplements that doctors believe may lower the risk of a certain type of cancer. These trials look for the best way to prevent cancer in people who have never had cancer or to prevent cancer from coming back or a new cancer from occurring in people who have already had cancer.
Screening trials test the best way to find cancer, especially in its early stage.
Quality of Life trials (also called Supportive Care trials) explore ways to improve comfort and quality of life for cancer patients.
What are the phases of clinical trials?
Most clinical research that involves the testing of a new drug progresses in an orderly series of steps, called phases. This allows researchers to ask and answer questions in a way that results in reliable information about the drug and protects the patients. Most clinical trials are classified into one of three phases:
Phase I: These first studies in people evaluate how a new drug should be given (by mouth, injected into the blood, or injected into the muscle), how often, and what dose is safe. A phase I trial usually enrols only a small number of patients, sometimes as few as a dozen.
Phase II: A phase II trial continues to test the safety of the drug, and begins to evaluate how well the new drug works. Phase II studies usually focus on a particular type of cancer.
Phase III: These studies test a new drug, a new combination of drugs, or a new surgical procedure in comparison to the current standard. A participant will usually be assigned to the standard group or the new group at random (called randomisation). Phase III trials often enrol large numbers of people and may be conducted at many doctors’ offices, clinics, and cancer centres nationwide.
Phase IV trial: The purpose of phase IV trials is to evaluate the side effects, risks, and benefits of a drug over a longer period of time and in a larger number of people than in phase III clinical trials. Thousands of people are involved in a phase IV trial.
Patients
What are Patient rights?
As a patient of CRSA, you have the right to:
- Understand and use these rights. If for any reason you do not understand or you need help, the group must provide assistance.
- Receive treatment without discrimination as to race, colour, religion, sex, national origin, disability, sexual orientation, or source of payment.
- Receive considerate and respectful care in a clean and safe environment free of unnecessary restraints.
- Receive emergency care if you need it.
- Be informed of the name and position of the doctor who will be in charge of your care.
- Know the names, positions, and functions of any staff involved in your care and refuse their treatment, examination, or observation.
- Receive complete information about your diagnosis, treatment, and prognosis.
- Receive all the information you need to give informed consent for any proposed procedure or treatment. This information shall include the possible risks and benefits of the procedure or treatment.
- Receive all the information you need to give informed consent for an order not to resuscitate. You also have the right to designate an individual to give this consent for you if you are too ill to do so.
- Refuse treatment and be told what effect this may have on your health.
- Refuse to take part in research. In deciding whether or not to participate, you have the right to a full explanation.
- Privacy and confidentiality of all information and records regarding your care.
- Participate in all decisions about your treatment and discharge from the hospital.
- Review your medical record after complying with current “Freedom of Information” legislation and obtain a copy of your medical record for which you will be charged a reasonable fee.
- Receive an itemized bill and explanation of all charges.
- Complain without fear of reprisals about the care and services you are receiving and to have the hospital respond to you, and if you request it, a written response.
What are Patient responsibilities?
When you are a patient of CRSA, it is your responsibility to:
- Provide accurate and complete information about your past illnesses, hospitalizations, medications, and other matters related to your health.
- Tell your physician or nurse if you do not understand your treatment.
- Inform your physician or nurse if there is a change in your condition or if problems arise during your treatment.
- Provide accurate information related to insurance or other sources of payment. Patients are responsible for assuring prompt payment of their bills. Tell us if you are having financial problems so that we may assist you in a timely manner.
- Be courteous and considerate of other patients and of staff.
Safety and quality
Quality in healthcare means the best possible health outcomes given the available circumstances and resources, consistent with patient centred care.
Safety in healthcare is reducing the risk of unnecessary harm to an acceptable minimum level. Patient safety is the freedom from hazards due to medical care or medical error in the practice setting. Harm can arise in healthcare, by omission or commission, and from the environment in which the healthcare is carried out.
In reality, the total absence of harm in the healthcare setting is unachievable and so the concept of safety relates to reducing the risk of unnecessary harm to an acceptable minimum level. An acceptable minimum level refers to the level of risk that is generally acceptable given the level of current knowledge, available resources and the context in which care is delivered weighed against the risk of having or not having treatment (RACGP 2016).
CRSA is committed to ongoing improvement of patient care in all areas. We have an excellent record in delivering quality patient care and managing risks, our centre continues to focus on improvements to ensure that our services are safe and that we are minimising risks at all times.
We have a strong commitment to safety and quality and this is reflected in our approach to:
- Creating a safe work place environment;
- Continuous review of work place practices
- Reviewing and improving our patient safety and quality systems;
- Assisting all our health care professionals to monitor the safety and quality of care they provide;
- Ensuring accountability for the safety and quality of care at all levels of our organisation
CRSA operates under a framework based on an integrated approach to clinical risk management and continuous quality improvement.
This framework measures four major areas of organisational performance including:
Risk Management
- Our culture promotes and encourages staff to report incidents, risks and near misses;
- Incident Management policy outlines the process for assessing and investigating incidents;
- Clinical policies are developed in accordance with evidence based best practice;
- Clinical, risk and safety policies are reviewed on a regular basis and updated as required.
Clinical Effectiveness
- Quality and Safety Indicators are used to measure and monitor performance;
- Quality plans are initiated when significant issues are flagged;
- Serious clinical incidents are reported and investigated;
- High risk areas are audited on a regular basis;
- Our facilities meet the standards for accreditation by ACHS/ISO.
Effective Workforce
- Ensuring a strict process for checking credentials, registration and scope of practice for all clinical disciplines;
- Targeted education and competency requirements in all clinical areas with a particular focus on high risk areas;
- Staff orientated and updated on quality and risk systems.
Consumer Participation
- Consumers participate in our risk management and quality improvement activities;
- Consumer complaints and feedback processes are managed in a timely manner;
- Consumer feedback from patient surveys;
- Consumers participate in improving patient experiences and health outcomes;
- Open disclosure between clinicians and consumers is promoted.
